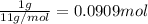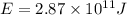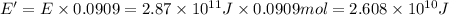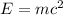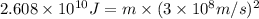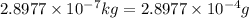Chemistry, 10.07.2019 09:00 ballin3294
Carbon-11 decays by positron emission: 116c → 115b + 01e the decay occurs with a release of 2.87 ⋅ 1011 j per mole of carbon-11. when 1.00 g of carbon-11 undergoes this radioactive decay, g of mass is converted to energy.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3


Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Carbon-11 decays by positron emission: 116c → 115b + 01e the decay occurs with a release of 2.87 ⋅...
Questions in other subjects:




World Languages, 10.10.2021 14:00

Biology, 10.10.2021 14:00




Geography, 10.10.2021 14:00

English, 10.10.2021 14:00

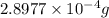 of mass is converted to energy.
of mass is converted to energy.