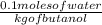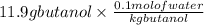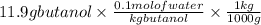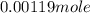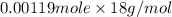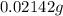Chemistry, 10.07.2019 09:00 sullivanjakob
The freezing point (tf) for t-butanol is 25.50°c and kf is 9.1°c/m. usually t-butanol absorbs water on exposure to the air. if the freezing point of a 11.9-g sample of t-butanol is measured as 24.59°c, how many grams of water are present in the sample?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:00, anonymous1813
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

You know the right answer?
The freezing point (tf) for t-butanol is 25.50°c and kf is 9.1°c/m. usually t-butanol absorbs water...
Questions in other subjects:



Mathematics, 12.11.2020 01:00




English, 12.11.2020 01:00

Mathematics, 12.11.2020 01:00


Mathematics, 12.11.2020 01:00

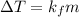 (1)
(1) = depression in freezing point
= depression in freezing point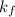 = molal freezing point.
= molal freezing point.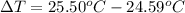
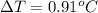
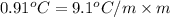
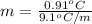
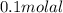 or
or