

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 07:00, kotetravels10
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
Lead(ii) iodide was prepared by reacting 65.0 ml of 0.218 m pb(no3)2 with 80.0 ml of 0.265 m ki. if...
Questions in other subjects:


Mathematics, 17.11.2020 20:10


English, 17.11.2020 20:10


Geography, 17.11.2020 20:10

Mathematics, 17.11.2020 20:10

Business, 17.11.2020 20:10


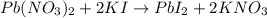
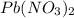 and KI, number of moles can be calculated as follows:
and KI, number of moles can be calculated as follows: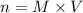
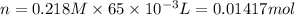
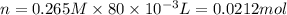
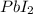 thus, 0.01417 mol will give 0.01417 mol.
thus, 0.01417 mol will give 0.01417 mol.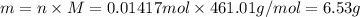
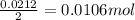 of
of 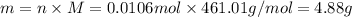
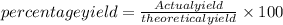
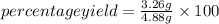 =66.80%
=66.80%

