

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
What is the molarity of a 5.39 m solution of benzene (c 6 h 6 ) dissolved in toluene (c 7 h 8 ),...
Questions in other subjects:


Biology, 04.10.2020 15:01


Mathematics, 04.10.2020 15:01


Mathematics, 04.10.2020 16:01


Mathematics, 04.10.2020 16:01

English, 04.10.2020 16:01

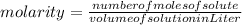 -(1)
-(1)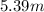 (given)
(given)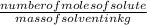
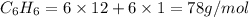
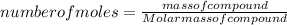 we can determine the mass of benzene as:
we can determine the mass of benzene as: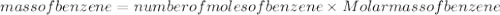
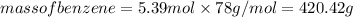
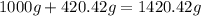
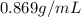 (given)
(given)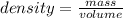
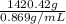 =
= 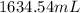
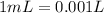
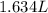
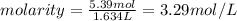
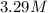 .
.

