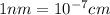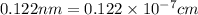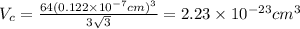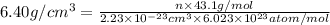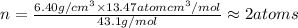Chemistry, 10.07.2019 12:30 savannahvargas512
Ahypothetical alloy has an atomic weight of 43.1 g/mol, a density of 6.40 g/cm3, and an atomic radius of 0.122 nm. determine whether its crystal structure is fcc, bcc, or simple cubic

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 23.06.2019 03:30, damyonfenton13
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

You know the right answer?
Ahypothetical alloy has an atomic weight of 43.1 g/mol, a density of 6.40 g/cm3, and an atomic radiu...
Questions in other subjects:

Mathematics, 20.07.2019 03:00

Geography, 20.07.2019 03:00






Mathematics, 20.07.2019 03:00

Mathematics, 20.07.2019 03:00

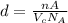 ...... (1)
...... (1)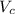 is volume of crystal and
is volume of crystal and 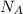 is Avagadro's number.
is Avagadro's number.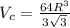
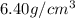 and 0.122 nm respectively.
and 0.122 nm respectively.