
Chemistry, 10.07.2019 13:00 iamsecond235p318rq
What mass of salt (nacl) should you add to 1.42 l of water in an ice cream maker to make a solution that freezes at -11.2 ∘c ? assume complete dissociation of the nacl and density of 1.00 g/ml for water?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2


Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1
You know the right answer?
What mass of salt (nacl) should you add to 1.42 l of water in an ice cream maker to make a solution...
Questions in other subjects:


Physics, 19.11.2020 22:00

Social Studies, 19.11.2020 22:00

Mathematics, 19.11.2020 22:00


Mathematics, 19.11.2020 22:00





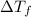 = depression in freezing point
= depression in freezing point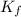 = molal depression constant (
= molal depression constant (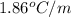 )
)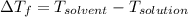
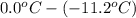
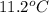
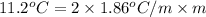 (as sodium chloride dissociate into two ions, i =2)
(as sodium chloride dissociate into two ions, i =2)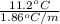
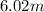

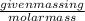
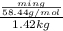 (1 L = 1kg)
(1 L = 1kg)




