Chemistry, 10.07.2019 13:30 lilyrockstarmag
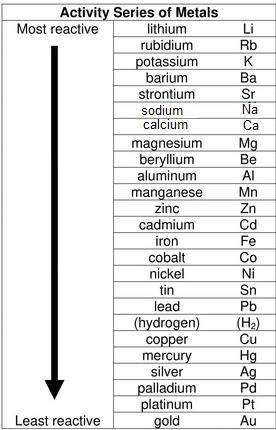
Which reaction will form products that are more stable than the reactants? a. 2albr3 + 3zn → 3znbr2 + 2al b. cabr2 + 2na → 2nabr + ca c. mgbr2 + h2 → 2hbr + mg d. babr2 + ca → cabr2 + ba e. 2libr + ba → babr2 + 2li

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Which reaction will form products that are more stable than the reactants? a. 2albr3 + 3zn → 3znbr2...
Questions in other subjects:

Mathematics, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50



Social Studies, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

Chemistry, 12.12.2020 15:50

Biology, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

Biology, 12.12.2020 15:50