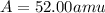Chemistry, 10.07.2019 16:30 jmurguia888
Acertain element x has four isotopes. 4.350% of x has a mass of 49.94605 amu. 83.79% of x has a mass of 51.94051 amu. 9.500% of x has a mass of 52.94065 amu. 2.360% of x has a mass of 53.93888 amu. what is the average atomic mass of element x? express your answer numerically to four significant figures. view available hint(s)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3


Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 23.06.2019 11:30, elizebeth4501
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1
You know the right answer?
Acertain element x has four isotopes. 4.350% of x has a mass of 49.94605 amu. 83.79% of x has a mass...
Questions in other subjects:

Health, 15.01.2020 04:31


Mathematics, 15.01.2020 04:31


History, 15.01.2020 04:31

Chemistry, 15.01.2020 04:31

Biology, 15.01.2020 04:31

English, 15.01.2020 04:31

English, 15.01.2020 04:31

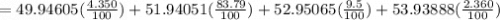
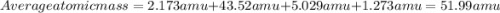
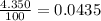
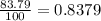
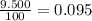
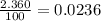
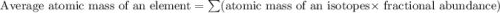
![A=\sum[(49.94605\times 0.0435)+(51.94051\times 0.8379)+(52.94065\times 0.095)+(53.93888\times 0.0236)]](/tpl/images/0073/8885/6e58e.png)