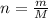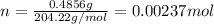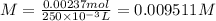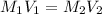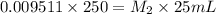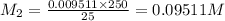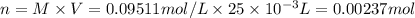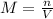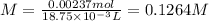Chemistry, 10.07.2019 16:30 bugsbunny27
If a 0.4856 gram sample of khp is dissolved in sufficient water to prepare 250 ml of solution, and 25 ml of the solution requires 18.76ml of sodium hydroxide solution to reach the equivalence point, what is the molarity of the sodium hydroxide?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
If a 0.4856 gram sample of khp is dissolved in sufficient water to prepare 250 ml of solution, and 2...
Questions in other subjects:

Spanish, 26.07.2019 05:00




Biology, 26.07.2019 05:00



English, 26.07.2019 05:00


Mathematics, 26.07.2019 05:00