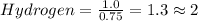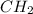Chemistry, 10.07.2019 16:30 hernan99961
Calculate the empirical formula of the hydrocarbon. combustion analysis of a hydrocarbon produced 33.01 g co2 and 9.02 g h2o.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

You know the right answer?
Calculate the empirical formula of the hydrocarbon. combustion analysis of a hydrocarbon produced 33...
Questions in other subjects:

Mathematics, 27.01.2021 14:00

Mathematics, 27.01.2021 14:00


Mathematics, 27.01.2021 14:00


Mathematics, 27.01.2021 14:00

Mathematics, 27.01.2021 14:00