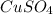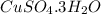Chemistry, 10.07.2019 21:00 saltedcaramel60
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of attached water molecules. after heating the hydrate, you have 3.3608 g of the anhydrous compound (copper(ii) sulfate with no waters) left. using these data, calculate the number of water molecules that is present in the formula of this hydrate (obviously before heating).

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2

You know the right answer?
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of a...
Questions in other subjects:

Mathematics, 01.10.2019 00:30

Social Studies, 01.10.2019 00:30

Mathematics, 01.10.2019 00:30



History, 01.10.2019 00:30


Mathematics, 01.10.2019 00:30


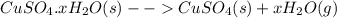
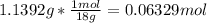
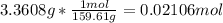
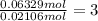
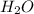 per one mol
per one mol