Chemistry, 10.07.2019 21:30 rmcarde3453
Asample of pure calcium fluoride with a mass of 15.0 g contains 7.70 g of calcium. how much calcium is contained in 45.0 g of calcium fluoride?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
Asample of pure calcium fluoride with a mass of 15.0 g contains 7.70 g of calcium. how much calcium...
Questions in other subjects:



Mathematics, 10.03.2021 04:30

Mathematics, 10.03.2021 04:30

Mathematics, 10.03.2021 04:30

Mathematics, 10.03.2021 04:30

Chemistry, 10.03.2021 04:30



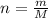
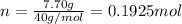
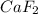 is 78.07 g/mol thus, number of moles will be:
is 78.07 g/mol thus, number of moles will be: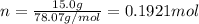 .
.