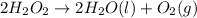During an experiment a thermometer was placed in a beaker containing hydrogen peroxide. the following observations were recorded when yeast granules were added to hydrogen peroxide. observation 1: fizzing and bubbling took place. observation 2: the temperature began to rise. based on the observation, justify the type of change (physical or chemical) that took place.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
During an experiment a thermometer was placed in a beaker containing hydrogen peroxide. the followin...
Questions in other subjects:



Social Studies, 23.06.2019 05:30



Spanish, 23.06.2019 05:30




Chemistry, 23.06.2019 05:30