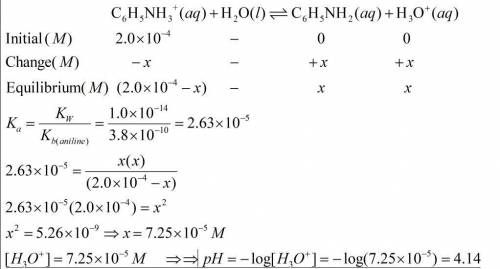Chemistry, 11.07.2019 02:30 QwesiElorm
A) calculate the ph of a 2.0x10-4 m solution of aniline hydrochoride, c6h5nh3cl.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 10:30, malum2009
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2

Chemistry, 23.06.2019 15:30, Akkenson17871
Which answer below correctly identifies the type of change and the explanation when magnesium comes into contact with hydrochloric acid
Answers: 1

Chemistry, 24.06.2019 03:40, Lovebamagirl12
How does the equilibrium change with the removal
Answers: 1
You know the right answer?
A) calculate the ph of a 2.0x10-4 m solution of aniline hydrochoride, c6h5nh3cl....
Questions in other subjects:


Mathematics, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

Biology, 10.09.2020 02:01

Arts, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

Arts, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

History, 10.09.2020 02:01


 anilinium ion the conjugate acid of aniline.
anilinium ion the conjugate acid of aniline.