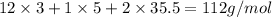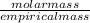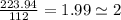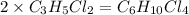Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, JotaroKujo6233
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1


Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
An unknown compound with a molar mass of 223.94 g/mol consists of 32.18% c, 4.50% h, and 63.32% cl....
Questions in other subjects:



Mathematics, 08.12.2021 22:00



Business, 08.12.2021 22:00

Mathematics, 08.12.2021 22:00

Mathematics, 08.12.2021 22:00


English, 08.12.2021 22:00

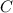 :
: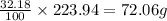
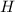 :
: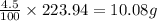
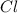 :
: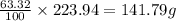
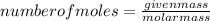
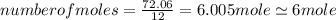
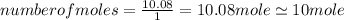
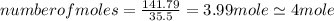
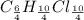
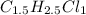
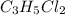 .
.