
Chemistry, 11.07.2019 07:00 baeethtsadia
Acommon anesthetic in dentistry known as nitronox consists of a mixture of dinitrogen oxide, n2o, and oxygen gas, o2, which is administered through an inhaler over the nose. if the anesthetic mixture has a total pressure of 740 mmhg, and the partial pressure of the oxygen is 370 mmhg, what is the partial pressure, in torr, of the dinitrogen oxide?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Acommon anesthetic in dentistry known as nitronox consists of a mixture of dinitrogen oxide, n2o, an...
Questions in other subjects:

Biology, 14.07.2019 03:30

Mathematics, 14.07.2019 03:30

Mathematics, 14.07.2019 03:30

Physics, 14.07.2019 03:30


Social Studies, 14.07.2019 03:30

Mathematics, 14.07.2019 03:30



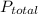 ) is sum of partial pressure of N₂O (
) is sum of partial pressure of N₂O (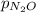 ) and partial pressure of O₂, (
) and partial pressure of O₂, (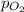 ).
).
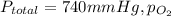 =370 mmHg
=370 mmHg


