
Chemistry, 11.07.2019 07:00 kaylamaisonettt
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 4.15 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 11:00, blondieb1722
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2
You know the right answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 4.15 ∘c. what is the molal concentration...
Questions in other subjects:






Mathematics, 28.02.2020 04:20


Mathematics, 28.02.2020 04:20


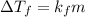 -(1)
-(1)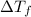 is depression of freezing point,
is depression of freezing point, 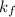 is freezing point depression constant and
is freezing point depression constant and 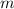 is molality.
is molality.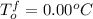 (given)
(given)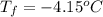 (given)
(given)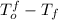
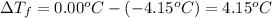
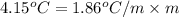
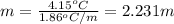
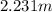 .
.

