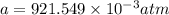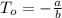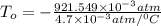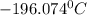Chemistry, 11.07.2019 07:00 lauren21bunch
In a student experiment, a constant-volume gas thermometer is calibrated in liquid nitrogen (−196°c ) and in boiling ethyl alcohol (77°c). the separate pressures are 0.349 atm and 1.634 atm. hint: use the linear relationship p = a + bt, where a and b are constants. (a) what value of absolute zero does the calibration yield?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 16:30, mbonham481
Amodel of an atom is shown below. which element is represented by this model of an atom? boron, carbon, neon, or sodium?
Answers: 1

Chemistry, 23.06.2019 19:00, tiffanibell71
Which law was used to determine the relationship between the volume and the number of moles in this equation
Answers: 2

Chemistry, 23.06.2019 21:30, gransazer863
Acertain substance has a solubility of 12 grams in 100 grams of water at 20°c. this means that the substance will begin to dissolve when 12 grams are present in solution when 12 grams of the substance are stirred into a beaker with 100 g of water, it will begin settling at the bottom when 12 g of the substance are dissolved in 100 grams of water, the solution will be dilute when 12 g of the substance are dissolved in 100 grams of water, the solution will be saturated
Answers: 1

Chemistry, 23.06.2019 22:50, joseperez1224
Compared with its corresponding unsaturated fatty acid, a saturated fatty acid has more hydrogen less hydrogen more oxygen less oxygen
Answers: 3
You know the right answer?
In a student experiment, a constant-volume gas thermometer is calibrated in liquid nitrogen (−196°c...
Questions in other subjects:

Mathematics, 12.03.2020 23:01

Mathematics, 12.03.2020 23:01

History, 12.03.2020 23:01


Mathematics, 12.03.2020 23:01


Mathematics, 12.03.2020 23:02


Mathematics, 12.03.2020 23:02

Mathematics, 12.03.2020 23:02

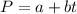
 (Pressure of liquid nitrogen) =
(Pressure of liquid nitrogen) =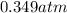
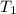 (Temperature of liquid nitrogen) =
(Temperature of liquid nitrogen) =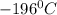
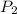 (Pressure of ethyl alcohol) =
(Pressure of ethyl alcohol) =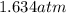
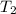 (Temperature of ethyl alcohol) =
(Temperature of ethyl alcohol) =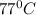
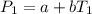
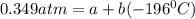 (1)
(1)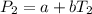
 (2)
(2)