
Chemistry, 11.07.2019 11:00 hflores0001
Chloe t. followed the procedure of this experiment to determine the empirical formula of a compound of iron (fe) and chlorine (cl). to do so, she added 2.15 g of zn to a solution containing 1.750 g of fe(x)cl(y). after the reaction was complete, she isolated 0.771 g of fe. the mass of cl in the fe(x)cl(y) solution is

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 22.06.2019 23:30, adamgala3885
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Chloe t. followed the procedure of this experiment to determine the empirical formula of a compound...
Questions in other subjects:



Mathematics, 01.07.2019 21:30


Mathematics, 01.07.2019 21:30






 sample contains only the atoms of
sample contains only the atoms of  , andChlorine,
, andChlorine, 
 and
and  are,
are, 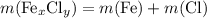 shall holds.
shall holds.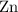 is more reactive than iron as seen in the reactivity series; Adding zinc to the solution would reduce all iron atoms (regardless of the oxidation state,
is more reactive than iron as seen in the reactivity series; Adding zinc to the solution would reduce all iron atoms (regardless of the oxidation state,  or
or  ) to their elementary form, hence displacing them out of the solution.
) to their elementary form, hence displacing them out of the solution. 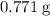 of iron was obtained and therefore
of iron was obtained and therefore  .
.



