
Chemistry, 11.07.2019 11:30 LindaCat78
Zinc metal reacts with hydrochloric acid according to the balanced equation: zn(s) + 2 hcl(aq) ¡ zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.5 °c to 23.7 °c. find ∆hrxn for this reaction as written. (use 1.0 g/ml for the

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1


Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Zinc metal reacts with hydrochloric acid according to the balanced equation: zn(s) + 2 hcl(aq) ¡ zn...
Questions in other subjects:

Physics, 18.09.2021 23:20


English, 18.09.2021 23:20


Mathematics, 18.09.2021 23:20

Mathematics, 18.09.2021 23:20




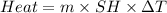
 is change in temperature.
is change in temperature.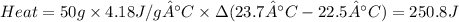
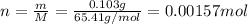
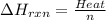
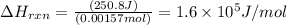
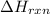 for the given reaction is
for the given reaction is 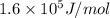
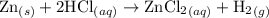
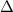 T,
T,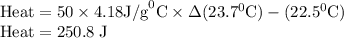
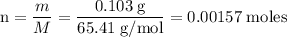
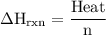
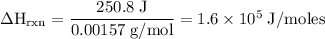
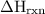 =
= 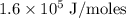 for the given equation.
for the given equation. 

