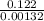Chemistry, 11.07.2019 11:30 taniyahbenyamin2
The decomposition reaction of a to b has a rate constant of 0.00132 s-1: a → 2b if the initial concentration of a is 0.156 m, how long will it take for the concentration of a to decrease by 78.1%

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
The decomposition reaction of a to b has a rate constant of 0.00132 s-1: a → 2b if the initial conc...
Questions in other subjects:


Health, 20.11.2020 04:10

History, 20.11.2020 04:10

Mathematics, 20.11.2020 04:10


Mathematics, 20.11.2020 04:10

Mathematics, 20.11.2020 04:10

Mathematics, 20.11.2020 04:10


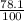 x 0.156
x 0.156