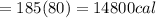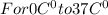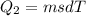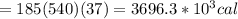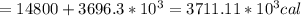Asports trainer applies an ice bag to the back of an injured athlete. calculate the heat in kcal that is absorbed if 185 g of ice at 0.0 ∘c is placed in an ice bag, melts, and rises to body temperature of 37.0 ∘ c. (for water, 80. cal (334 j) is needed to melt 1 g of ice or must be removed to freeze 1 g of water.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1


Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Asports trainer applies an ice bag to the back of an injured athlete. calculate the heat in kcal tha...
Questions in other subjects:

Mathematics, 10.12.2020 17:10



Mathematics, 10.12.2020 17:10





Mathematics, 10.12.2020 17:10