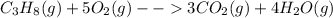During a chemical reaction, 25.0 g of propane burns with 90.7 g of oxygen to form carbon dioxide and water. what mass of carbon dioxide and water is formed? during a chemical reaction, 25.0 g of propane burns with 90.7 g of oxygen to form carbon dioxide and water. what mass of carbon dioxide and water is formed? 115.7 g 90.7 g 65.7 g 25.0 g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, mildredelizam
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
During a chemical reaction, 25.0 g of propane burns with 90.7 g of oxygen to form carbon dioxide and...
Questions in other subjects:

Mathematics, 08.01.2021 16:20



Arts, 08.01.2021 16:20

History, 08.01.2021 16:20

Mathematics, 08.01.2021 16:20

Chemistry, 08.01.2021 16:20

Mathematics, 08.01.2021 16:20

English, 08.01.2021 16:20