

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
A0.99 m aqueous solution of an ionic compound with the formula mx has a freezing point of -2.6 ∘c ....
Questions in other subjects:


Physics, 07.05.2020 11:03

Mathematics, 07.05.2020 11:03





Mathematics, 07.05.2020 11:03


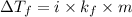 -(1)
-(1)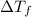 is depression in freezing point,
is depression in freezing point, is Van't Hoff factor,
is Van't Hoff factor,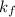 is molal freezing point depression constant, and
is molal freezing point depression constant, and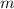 is molality of the solution.
is molality of the solution.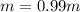 (given)
(given)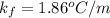
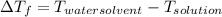
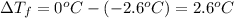
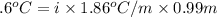
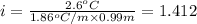
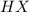 is 1.412.
is 1.412.


