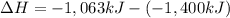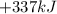Chemistry, 11.07.2019 19:30 Sadiecoyle37
What is the change in enthalpy for the single replacement reaction between solid zinc and cs2so4 in solution to produce cesium and znso4? zn + cs2so4 → 2cs + znso4 given: `"cs"_2"so"_4(aq): deltah_f °= –1,400 "kj"` `"znso"_4(aq): deltah_f °= –1,063 "kj"`

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, penelopymorales24
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
What is the change in enthalpy for the single replacement reaction between solid zinc and cs2so4 in...
Questions in other subjects:


Mathematics, 02.03.2021 21:10

Mathematics, 02.03.2021 21:10

Geography, 02.03.2021 21:10

History, 02.03.2021 21:10

Computers and Technology, 02.03.2021 21:10


Mathematics, 02.03.2021 21:10


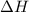 .
.
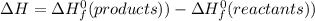
 (products) and
(products) and