
Chemistry, 11.07.2019 19:30 calhountoiyonou0gjb
You are given a 1.50 g mixture of sodium nitrate and sodium chloride. you dissolve this mixture into 100. ml of water and then add an excess of 0.500 m silver nitrate solution. the white solid produced from the reaction is filtered, dried and measured. the white solid has a mass of 0.641 g. calculate the percent, by mass, of sodium chloride in the original unknown mixture.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
You are given a 1.50 g mixture of sodium nitrate and sodium chloride. you dissolve this mixture into...
Questions in other subjects:




Mathematics, 15.04.2020 17:36






Mathematics, 15.04.2020 17:36

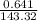 = 4.47 X
= 4.47 X 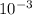 mole of solid AgCl is produced. This indicates 4.47 X
mole of solid AgCl is produced. This indicates 4.47 X 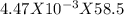 g of Nacl reacts to give 0.641 g of AgCl. So, mass percentage of NaCl present in mixture=
g of Nacl reacts to give 0.641 g of AgCl. So, mass percentage of NaCl present in mixture=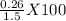 = 17.33 %.
= 17.33 %.

