

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1


You know the right answer?
Atechnician experimentally determined the boiling point of octane to be 124.1°c. the actual boiling...
Questions in other subjects:


Mathematics, 30.06.2019 09:00






Biology, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00


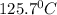
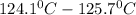





 .
.

