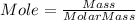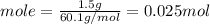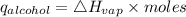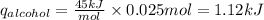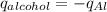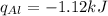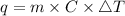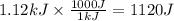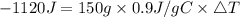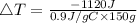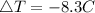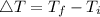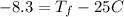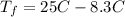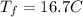Chemistry, 11.07.2019 22:30 reesewaggoner8
Consider 1.5g of rubbing alcohol (c3h8o) placed on a 150.g block of aluminum at 25 c. if all of the rubbing alcohol evaporates at 25 c, what is the final temperature of the aluminum?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
Consider 1.5g of rubbing alcohol (c3h8o) placed on a 150.g block of aluminum at 25 c. if all of the...
Questions in other subjects:

Biology, 17.06.2020 04:57





History, 17.06.2020 04:57