
Chemistry, 11.07.2019 23:30 steffweikeloyrt00
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of coffee with a temperature of 88.3 degrees c. assume the specific heats of coffee and milk are the same as for water (c = 4.19 j/g•c), and disregard the heat capacity of the container.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of co...
Questions in other subjects:


Mathematics, 09.09.2020 07:01





Mathematics, 09.09.2020 07:01


English, 09.09.2020 07:01

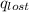 =
=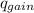 ,
,  +
+ 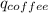
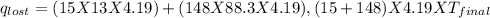 =(15X13X4.19)+(148X88.3X4.19)
=(15X13X4.19)+(148X88.3X4.19)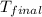 = 81.37 ° C
= 81.37 ° C



