
Chemistry, 12.07.2019 01:30 nefertiri64
In a reaction where a + b ab, what does an equilibrium position of a= 14%, b = 14%, and ab= 72% indicate about the reaction? a. because a and b are equal, the reaction has reached equilibrium. b. there is more a and b than ab at equilibrium, and the reaction favors the reactants. c. there is more ab than a and b at equilibrium, and the reaction favors the products. d. there is more ab than a and b at equilibrium, and the reaction favors the reactants. e. the reaction is not at equilibrium.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
In a reaction where a + b ab, what does an equilibrium position of a= 14%, b = 14%, and ab= 72% indi...
Questions in other subjects:


Mathematics, 09.12.2020 22:00



Mathematics, 09.12.2020 22:00




Mathematics, 09.12.2020 22:00

 is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.

![K_c=\frac{[AB]}{[A][B]}](/tpl/images/0079/1606/5faa1.png)
![K_c=\frac{[0.72]}{[0.14][0.14]}=36.7](/tpl/images/0079/1606/2d664.png)
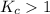 ; then the reaction is product favored.
; then the reaction is product favored.
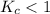 ; then the reaction is reactant favored.
; then the reaction is reactant favored.
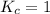 ; then the reaction is in equilibrium.
; then the reaction is in equilibrium.


