
Acompound contains only carbon, hydrogen, and oxygen. combustion of 9.612 mg of the compound yields 14.41 mg co2 and 3.93 mg h2o. the molar mass of the compound is 176.1 g/mol. what are the empirical and molecular formulas of the compound? (type your answer using the format co2 for co2.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 01:30, kaitie60
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
Acompound contains only carbon, hydrogen, and oxygen. combustion of 9.612 mg of the compound yields...
Questions in other subjects:

Mathematics, 23.11.2020 03:40


Mathematics, 23.11.2020 03:40

English, 23.11.2020 03:40






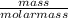 -(1)
-(1)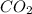 = 14.41 mg (given)
= 14.41 mg (given)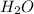 = 3.93 mg (given)
= 3.93 mg (given)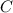 from
from 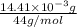
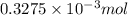
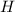 from
from 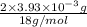
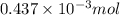
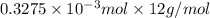
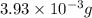
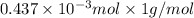
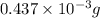
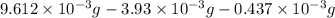
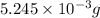
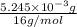
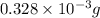
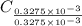
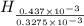
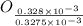
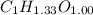
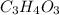
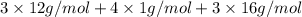
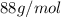
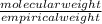 =
= 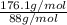
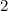
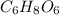 .
.

