
Chemists often use molarity m, in moles/liter, to measure the concentration of solutions. molarity is a common unit of concentration because the volume of a liquid is very easy to measure. however, the drawback of using molarity is that volume is a temperature-dependent quantity. as temperature changes, density changes, which affects volume. volume markings for most laboratory glassware are calibrated for room temperature, about 20∘c. fortunately, there are several other ways of expressing concentration that do not involve volume and are therefore temperature independent. a 2.800×10−2 m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample of nacl in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 999.2 ml . the density of water at 20.0∘c is 0.9982 g/ml.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1


Chemistry, 22.06.2019 19:50, strawberrymrmr756
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
Chemists often use molarity m, in moles/liter, to measure the concentration of solutions. molarity i...
Questions in other subjects:







Mathematics, 08.04.2020 03:10


Mathematics, 08.04.2020 03:10

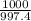 x 2.800×10⁻² moles of NaCl.
x 2.800×10⁻² moles of NaCl.


