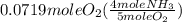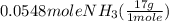Chemistry, 12.07.2019 02:30 Thejollyhellhound20
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion of nh3 to no. 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(g) in a certain experiment, 1.90 g of nh3 reacts with 2.30 g of o2. (a) which is the limiting reactant? o2 nh3 (b) how many grams of no and of h2o form? 1.73 g no 1.55 g h2o (c) how many grams of the excess reactant remain after the limiting reactant is completely consumed? g (d) show that your calculations in parts (b) and (c) are consistent with the law of conservation of mass. mass of products + excess reactant g total mass of reactants g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 21.06.2019 22:30, johnnydenali67
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion o...
Questions in other subjects:


Mathematics, 16.12.2020 22:00


Mathematics, 16.12.2020 22:00

Mathematics, 16.12.2020 22:00


Mathematics, 16.12.2020 22:00


Arts, 16.12.2020 22:00

History, 16.12.2020 22:00

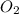 , (b) 1.73 g NO and 1.55 g
, (b) 1.73 g NO and 1.55 g 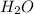 , (c) 0.932 g of
, (c) 0.932 g of 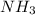 , (d) yes, the results are consistent with law of conservation of mass.
, (d) yes, the results are consistent with law of conservation of mass.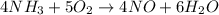
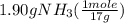 = 0.112 mole
= 0.112 mole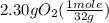 = 0.0719 mole
= 0.0719 mole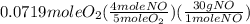 = 1.73 g of NO
= 1.73 g of NO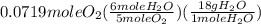 = 1.55 g of
= 1.55 g of