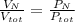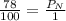Air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25.0 ∘c, the n2 component will dissolve in water with a solubility of 4.88×10−4 m. what is the value of henry's law constant for n2 under these conditions? express your answer with the appropriate units. enter the unit m using the compound form mol/l.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
Air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25....
Questions in other subjects:

History, 26.09.2019 04:00

English, 26.09.2019 04:00



Advanced Placement (AP), 26.09.2019 04:00

English, 26.09.2019 04:00


English, 26.09.2019 04:00

Mathematics, 26.09.2019 04:00

Health, 26.09.2019 04:00