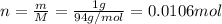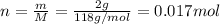Chemistry, 12.07.2019 05:30 risolatziyovudd
If 1.00 g of reactant a is mixed with 2.00 g of reactant b, what is the theoretical yield of product d, in grams (g)? the molar mass of a = 94.0 g/mol, b = 118 g/mol , and d = 125 g/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
If 1.00 g of reactant a is mixed with 2.00 g of reactant b, what is the theoretical yield of product...
Questions in other subjects:


Biology, 29.03.2021 22:30


French, 29.03.2021 22:30

History, 29.03.2021 22:30


Mathematics, 29.03.2021 22:30


Mathematics, 29.03.2021 22:30

Mathematics, 29.03.2021 22:30