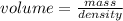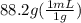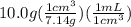Chemistry, 12.07.2019 08:30 nickboy52210
An empty flask has a mass of 123.4 g. when the flask is filled with water, the madd is 211.6g. if 10.0g of zinc (d=7.14 g/cm^3) are added to the flask filled with water (and the sides of the flask are dried from the displaced water) what is the new volume in the flask? (hint: you are going to have to use the density of water to answer this question)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1


Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
An empty flask has a mass of 123.4 g. when the flask is filled with water, the madd is 211.6g. if 10...
Questions in other subjects:

English, 21.12.2019 20:31

History, 21.12.2019 20:31


Physics, 21.12.2019 20:31

Biology, 21.12.2019 20:31


Mathematics, 21.12.2019 20:31

History, 21.12.2019 20:31

Biology, 21.12.2019 20:31

 .
.