
Chemistry, 12.07.2019 09:00 gracieorman4
Gaseous ammonia chemically reacts with oxygen o2 gas to produce nitrogen monoxide gas and water vapor. calculate the moles of water produced by the reaction of 0.060mol of oxygen. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1
You know the right answer?
Gaseous ammonia chemically reacts with oxygen o2 gas to produce nitrogen monoxide gas and water vapo...
Questions in other subjects:

Mathematics, 01.04.2020 01:27




Mathematics, 01.04.2020 01:27

Arts, 01.04.2020 01:27


English, 01.04.2020 01:27


English, 01.04.2020 01:27

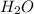 ) produced by the reaction of 0.060mol of oxygen.
) produced by the reaction of 0.060mol of oxygen.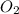 ) given = 0.060 mole
) given = 0.060 mole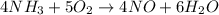
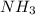 to give 4 moles of NO and 6 moles of
to give 4 moles of NO and 6 moles of 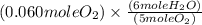
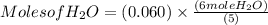
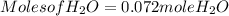
 4 NO + 6 H₂O
4 NO + 6 H₂O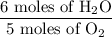 Moles of H₂O = 0.060 moles of O₂ x
Moles of H₂O = 0.060 moles of O₂ x 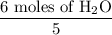 Moles of H₂O = 0.072 moles of H₂O.
Moles of H₂O = 0.072 moles of H₂O. 

