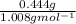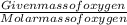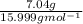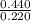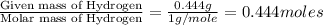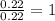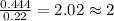Chemistry, 12.07.2019 09:00 cxttiemsp021
What is the empirical formula of a substance that contains 2.64 g of c, 0.444 g of h, and 7.04 g of o?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1


Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
What is the empirical formula of a substance that contains 2.64 g of c, 0.444 g of h, and 7.04 g of...
Questions in other subjects:


English, 11.03.2020 16:57





History, 11.03.2020 16:57


Mathematics, 11.03.2020 16:57