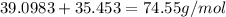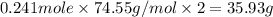Chemistry, 12.07.2019 09:00 mcdonaldmacy01
The compound cisplatin, pt(nh3)2cl2 , has been studied extensively as an antitumor agent. a. calculate the elemental percent composition by mass of cisplatin. b. cisplatin is synthesized as follows: k2ptcl4 (aq) + 2nh3(aq) â pt(nh3)2cl2 (s) + 2kcl (aq) what mass of cisplatin can be made from 100.g of k2ptcl4 and sufficient nh3? what mass of kcl is also produced?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

You know the right answer?
The compound cisplatin, pt(nh3)2cl2 , has been studied extensively as an antitumor agent. a. calcula...
Questions in other subjects:




Physics, 24.07.2019 19:30






History, 24.07.2019 19:30

 (1)
(1)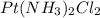 .
.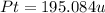
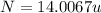
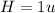
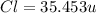
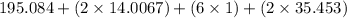 =
= 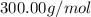
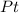 %
%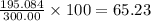 %.
%.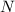 %
%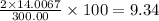 %
%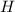 %
%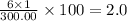 %
%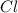 %
%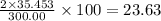 %
%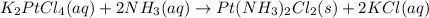
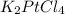 gives 1 mole of
gives 1 mole of 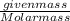
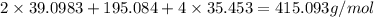
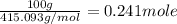 .
.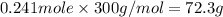
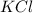 :
: