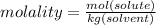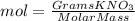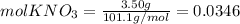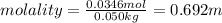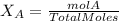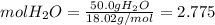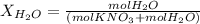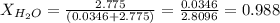Chemistry, 12.07.2019 12:00 Pizzapegasus1
Calculate the molality and mole fraction of water, respectively, of a solution that is made by dissolving 3.50 g of potassium nitrate in 50.0 g of water. the final volume of the solution is 56.0 ml.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1


Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
Calculate the molality and mole fraction of water, respectively, of a solution that is made by disso...
Questions in other subjects:

Business, 02.01.2020 23:31

Physics, 02.01.2020 23:31


Social Studies, 02.01.2020 23:31