
Chemistry, 12.07.2019 13:00 daebreonnakelly
Calculate the ph for the following weak acid. a solution of hcooh has 0.14m hcooh at equilibrium. the ka for hcooh is 1.8×10−4. what is the ph of this solution at equilibrium? express the ph numerically.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 22:10, preachersgirl5
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
Calculate the ph for the following weak acid. a solution of hcooh has 0.14m hcooh at equilibrium. th...
Questions in other subjects:



Social Studies, 14.01.2021 22:10

Biology, 14.01.2021 22:10


English, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

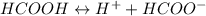
![Ka=\frac{[H^+][HCOO^-]}{[HCOOH]}](/tpl/images/0081/0231/b2152.png)
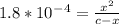
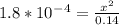
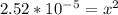
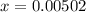
![[H^+]](/tpl/images/0081/0231/07acb.png) .
. ![pH=-log[H^+]](/tpl/images/0081/0231/15713.png)


