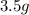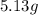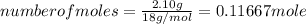Chemistry, 12.07.2019 15:00 jsully5159
An unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 3.50 g of this compound produced 5.13 g of carbon dioxide and 2.10 g of water. how many moles of hydrogen, h, were in the original sample?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
An unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 3.50 g of thi...
Questions in other subjects:


Arts, 14.11.2020 09:40


Mathematics, 14.11.2020 09:40

Mathematics, 14.11.2020 09:40

Mathematics, 14.11.2020 09:40

Mathematics, 14.11.2020 09:40



Health, 14.11.2020 09:40