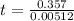Chemistry, 12.07.2019 18:00 JammedBanjo58
Consider the below chemical reaction that occurs via first-order kinetics with a rate constant of 5.12 x 10–3 s–1 at a particular temperature. how long will it take for 30% of substrate a to be consumed? a → b 83.7 s

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Consider the below chemical reaction that occurs via first-order kinetics with a rate constant of 5....
Questions in other subjects:

Mathematics, 24.03.2021 18:30

Social Studies, 24.03.2021 18:30

Mathematics, 24.03.2021 18:30


English, 24.03.2021 18:30

Arts, 24.03.2021 18:30




Mathematics, 24.03.2021 18:30

![ln[A]=-kt+ln[A_0]](/tpl/images/0081/8081/fcf7e.png)
![[A_0]](/tpl/images/0081/8081/9a686.png) is the initial concentration and [A] is final concentration.
is the initial concentration and [A] is final concentration.
![ln[70]=-5.12*10^-^3(t)+ln[100]](/tpl/images/0081/8081/d14d5.png)