
Chemistry, 12.07.2019 18:00 darkghostmist
The unknowns are a mixture of ferrous ammonium sulfate and ammonium sulfate. calculate the amount of ferrous ammonium sulfate and the amount of ammonium sulfate needed to prepare a sample that is 10% fe with a total mass of 0.2 g

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1

Chemistry, 23.06.2019 00:30, coralaguilar1702
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
The unknowns are a mixture of ferrous ammonium sulfate and ammonium sulfate. calculate the amount of...
Questions in other subjects:

Mathematics, 02.04.2021 17:10

Mathematics, 02.04.2021 17:10

Chemistry, 02.04.2021 17:10

English, 02.04.2021 17:10

Mathematics, 02.04.2021 17:10

Chemistry, 02.04.2021 17:10

History, 02.04.2021 17:10

Mathematics, 02.04.2021 17:10

Mathematics, 02.04.2021 17:10

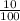 x0.2
x0.2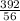 x 0.02
x 0.02

