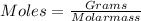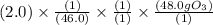Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Ozone, o3, is a product in automobile exhaust by the reaction represented by the equation no2(g) + o...
Questions in other subjects:

Mathematics, 26.11.2020 06:10



Advanced Placement (AP), 26.11.2020 06:10

Physics, 26.11.2020 06:10

Geography, 26.11.2020 06:10


Business, 26.11.2020 06:10

Arts, 26.11.2020 06:10

Social Studies, 26.11.2020 06:10

 ) is predicted to form from the reaction of 2.0 g
) is predicted to form from the reaction of 2.0 g  in a car's exhaust and excess oxygen
in a car's exhaust and excess oxygen is in excess.
is in excess.