
Chemistry, 12.07.2019 21:00 kotetravels10
Asample of an unknown substance contains 38.43 g lead, 17.83 g carbon, and 3.74 g hydrogen. what is the empirical formula? a: pbc4h10 b: pb2c8h15 c: pbc8h20 d: pb2c4h15

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1


Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Asample of an unknown substance contains 38.43 g lead, 17.83 g carbon, and 3.74 g hydrogen. what is...
Questions in other subjects:



History, 26.09.2019 12:50


Biology, 26.09.2019 12:50





History, 26.09.2019 12:50

 .
.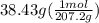


 = 1
= 1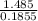 = 8
= 8 = 20
= 20

