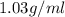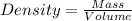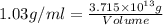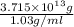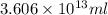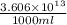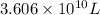Chemistry, 12.07.2019 22:00 jewelz5887
Magnesium (mg) is a valuable metal used in alloys, in batteries, and in the manufacture of chemicals. it is obtained mostly from seawater, which contains about 1.30 g of mg for every kilogram of seawater. calculate the volume of seawater (in liters) needed to extract 5.33 × 104 tons of mg. seawater has a density of 1.03 g/ml. (1 ton = 2000 lb; 1 lb = 453.6 g) enter your answer in scientific notation.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


You know the right answer?
Magnesium (mg) is a valuable metal used in alloys, in batteries, and in the manufacture of chemicals...
Questions in other subjects:

Mathematics, 19.04.2020 03:45



History, 19.04.2020 03:45


Health, 19.04.2020 03:45


Biology, 19.04.2020 03:46

Biology, 19.04.2020 03:46

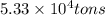 into gram.
into gram.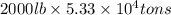
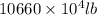 =
= 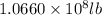
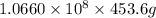
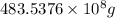 =
= 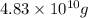
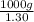 of seawater.
of seawater.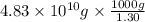 =
= 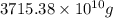
 of seawater.
of seawater.