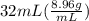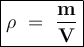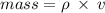Chemistry, 12.07.2019 22:30 annette211pdd8v9
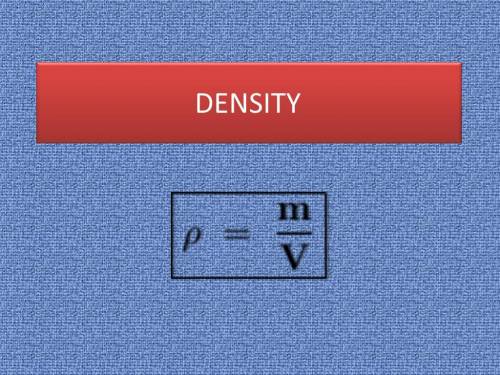
You are asked to determine the mass of a piece of copper using its reported density, 8.96 g/ml, and a 150-ml graduated cylinder. first, you add 105 ml of water to the graduated cylinder; then you place the piece of copper in the cylinder and record a volume of 137 ml. what is the mass of the copper reported with the correct number of significant figures?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1

Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
You are asked to determine the mass of a piece of copper using its reported density, 8.96 g/ml, and...
Questions in other subjects:


Mathematics, 05.10.2021 05:20

Mathematics, 05.10.2021 05:20

English, 05.10.2021 05:20



Mathematics, 05.10.2021 05:20

Social Studies, 05.10.2021 05:20

Mathematics, 05.10.2021 05:20

Computers and Technology, 05.10.2021 05:20