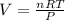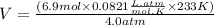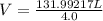Chemistry, 13.07.2019 00:30 Isaiahtate053
What is the volume of 6.9 mol oxygen (o2) gas at 233 k and a pressure of 4.0 atm? (the universal gas constant is 0.0821 l•atm/mol•k.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1


Chemistry, 22.06.2019 05:30, smartie80
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3
You know the right answer?
What is the volume of 6.9 mol oxygen (o2) gas at 233 k and a pressure of 4.0 atm? (the universal ga...
Questions in other subjects:

Biology, 06.01.2020 18:31


Chemistry, 06.01.2020 18:31

Mathematics, 06.01.2020 18:31



Social Studies, 06.01.2020 18:31