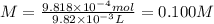Chemistry, 13.07.2019 00:30 tookie6208
In part 1 of lab 2 you will make and standardize a solution of naoh(aq). suppose in the lab you measure the solid naoh and dissolve it into 100.0 ml of water. you then measure 0.2005 g of khp (kc8h5o4, 204.22 g/mol) and place it in a clean, dry 100-ml beaker, and then dissolve the khp in about 25 ml of water and add a couple of drops of phenolphthalein indicator. you titrate this with your naoh(aq) solution and find that the titration requires 9.82 ml of naoh(aq).

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, MrSavannahCat
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
In part 1 of lab 2 you will make and standardize a solution of naoh(aq). suppose in the lab you meas...
Questions in other subjects:

Mathematics, 26.02.2021 22:50


English, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50


Biology, 26.02.2021 22:50




Biology, 26.02.2021 22:50